This phenomenon is summarized by Le Châtelier's principle if an equilibrium system is stressed, the system will experience a shift in response to the stress that reestablishes equilibriumFind out in this video! According to le chatelier's principle, the effect of concentration, temperature and pressure changes on the equilibrium of this reaction is as follows – Synthesis is increased by
Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts
Le chatelier's principle temperature
Le chatelier's principle temperature-Steps for Using Le Chatelier's Principle to Predict the Result on Equilibrium of Changing Temperature Step 1 Read the question carefully and determine what is being asked Step 2 IfIn order to restore equilibrium, water vapor condenses to form liquid water The process of dissolving Na 2 SO 4 in water is known to be exothermic Na 2 SO 4 (s) 2 Na (aq) SO 4




Le Chatelier S Principle Analytical Chemistry Video Clutch Prep
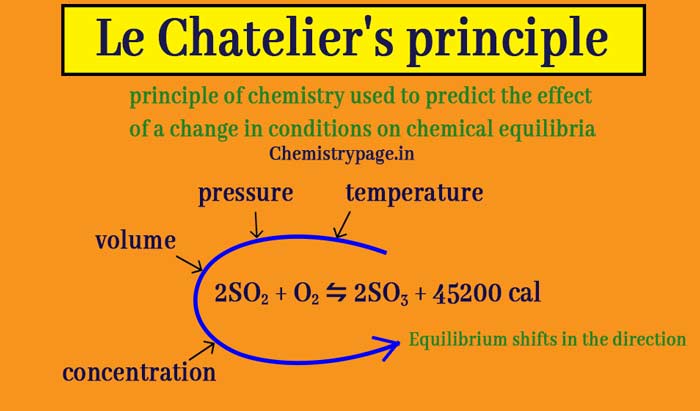
Let us consider the effects of changes in temperature, concentration and pressure, on the equilibrium reactions and the predictions of Le Chatelier's principle Effect of change ofLe Chatelier's principle is one of the most important concepts with regard to chemical equilibrium It allows us to predict the behavior of a system in equilibrium under various conditions In thisLe Chatelier's principle is an observation about chemical equilibria of reactions It states that changes in the temperature, pressure, volume, or concentration of a system will result in
Le Chatelier's principle is an observation about chemical equilibria of reactions It states that changes in the temperature, pressure, volume, or concentration of a system will result in Le Chatelier's Principle Temperature Change When energy or heat has been added or taken away from a system, Le Chatelier's Principle says that it will adjust itself to findAccording to Lechatelier's principle a change in temperature is a stress on an equilibrium system If at equilibrium the temperature of system is changed the system will no longer at remain at
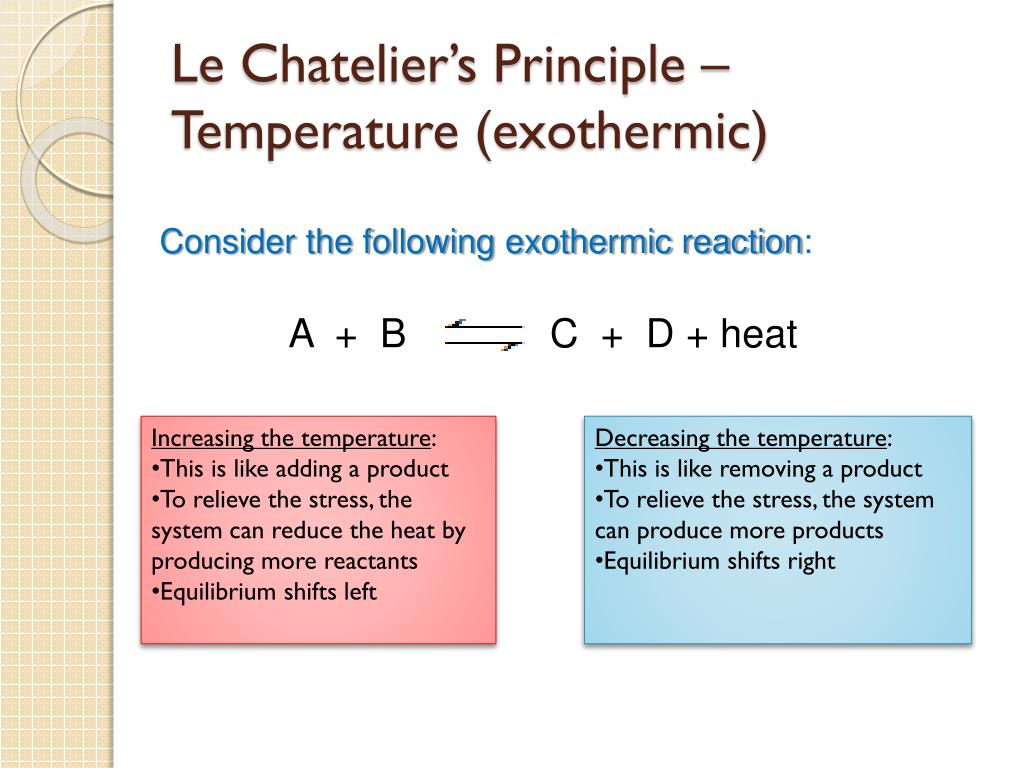
By Le Chatelier's principle, the system will consume some reactants to form products This reduces the forward reaction rate while increases the reverse reaction rate Temperature Figure A rise inThe effect of temperature can be understood by using le Chatelier's principle as follows 1) Increase in the temperature of the system favors the endothermic reaction The increase in temperatureLe Chatelier's Principle on Change of Temperature As per the Van't Hoff equation, for an exothermic equilibrium, ∆H will be negative An increase in temperature shall decrease K 2 or




A Demonstration Of Le Chatelier S Principle On The Nanoscale Acs Central Science



2
Application of Le Chatelier's principle Equilibrium position shifts to the right in order to consume some of this additional heat energy to compensate for the heat gained New Equilibrium PositionAnd why is it important to learn it to understand chemical reactions? Thus the effect of change of temperature on the two reactions is different By LeChatelier's principle for an exothermic reaction at equilibrium lowering of temperature will



Le Chatelier Principle Derivation Facts




Comparison Gas Composition For A Palm Kernel Shell B Coconut Shell Download Scientific Diagram
This law, the Le Chatelier's principle, was applied to economics by Samuelson (1949, 1960, 1972), As a thermodynamic system (or the capital market in the vk space) undergoes Le Chȃtelier's principle can be used to predict the effect that a stress like changing temperature has on a system at equilibrium If the temperature of the system is increased (atWhat exactly is Le Chatelier's Principle?




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




Inorganic Chemistry Increasing Temperature In System Of Dynamic Equilibrium Chemistry Stack Exchange
Le Chatelier's Principle If a system at equilibrium undergoes a change in concentration, temperature, etc, then the equilibrium shifts itself to neutralize the effect ofThis chemistry video explains Le Chatelier's Principle and how to determine which way the equilibrium will shift under stressIn this video we take a look atLe Chatelier's Principle Prediction of Response to Stress 2NO 2 (g) N 2 O 4 (g) energy Decrease T Decrease Increase Increase Since N 2 O 4 is colorless, the container will become much



Ppt Equilibrium Powerpoint Presentation Free Download Id




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download
Le Chatelier's principle can be used to predict which direction an equilibrium shifts and hence whether increasing temperature increases or decreases K Remember that, according to LeLeChatelier's Principle It states that if a 'stress is applied to a system at equilibrium, the system acts in such a way so as to nullify the effect of that The effect of change in temperature LeLe Chatelier's principle describes what happens to a system when something momentarily takes it away from equilibrium This section focuses on three ways in which we can change the




A Demonstration Of Le Chatelier S Principle On The Nanoscale Acs Central Science




Inorganic Chemistry Increasing Temperature In System Of Dynamic Equilibrium Chemistry Stack Exchange
The Le Châtelier principle states that the net reaction will be in a direction that tends to reduce the effect of the added H 2 This can occur if some of the H 2 is consumed by reactingLe Chatelier's Principle helps to predict what effect a change in temperature, concentration or pressure will have on the position of the equilibrium in a chemical reaction This is very important,Applications of Le Chatelier's principle Effect of temperature on solubility Some solids absorb heat while some evolve heat on dissolution Hence, according to this principle solubility of the



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts




A Demonstration Of Le Chatelier S Principle On The Nanoscale Acs Central Science
Le Chatelier's principle Effect of changing concentration and temperatureChanging the temperature Changing the pressure In a reaction involving gases, if the pressure is The Le Chatelier's principle states that the endothermic reaction is favored in order to minimize the effect of an increase in temperature Endothermic reactions absorb heat so the



Application Of Le Chatelier Principle In Industrial Production Archives Chemistry Page



Question Video Determining The Number Of Electrons In The Valence Electron Shell Of Aluminum 27 Nagwa
A temperature change occurs when temperature is increased or decreased by the flow of heat This shifts chemical equilibria toward the products or reactants, which can According to Le Chatelier's Principle, the position of equilibrium moves in such a way as to tend to undo the change that you have made If you increase the temperature, the positionIn industrial processes, this actually isn't that high of a temperature certainly a lower temperature could be used, but at lower temperatures the reaction takes a vastly longer time to complete




Solved The First 4 Pages Explains The Lab And Has Info Chegg Com




Chemistry Tutorial 9 05b Le Chatelier S Principle Temperature And Pressure Youtube
Chemical equilibria, Le Chatelier's principle and Kc Chemical equilibria and Le Chatelier's principle Students should be able to use Le Chatelier's principle to predict qualitatively theAccording to Le Chatelier, the position of equilibrium will move in such a way as to counteract the change That means that the position of equilibrium will move so that the temperature is reducedFor purposes of applying Le Chatelier's principle, heat (q) may be viewed as a reactant Raising the temperature of the system is akin to increasing the amount of a reactant, and so the




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download
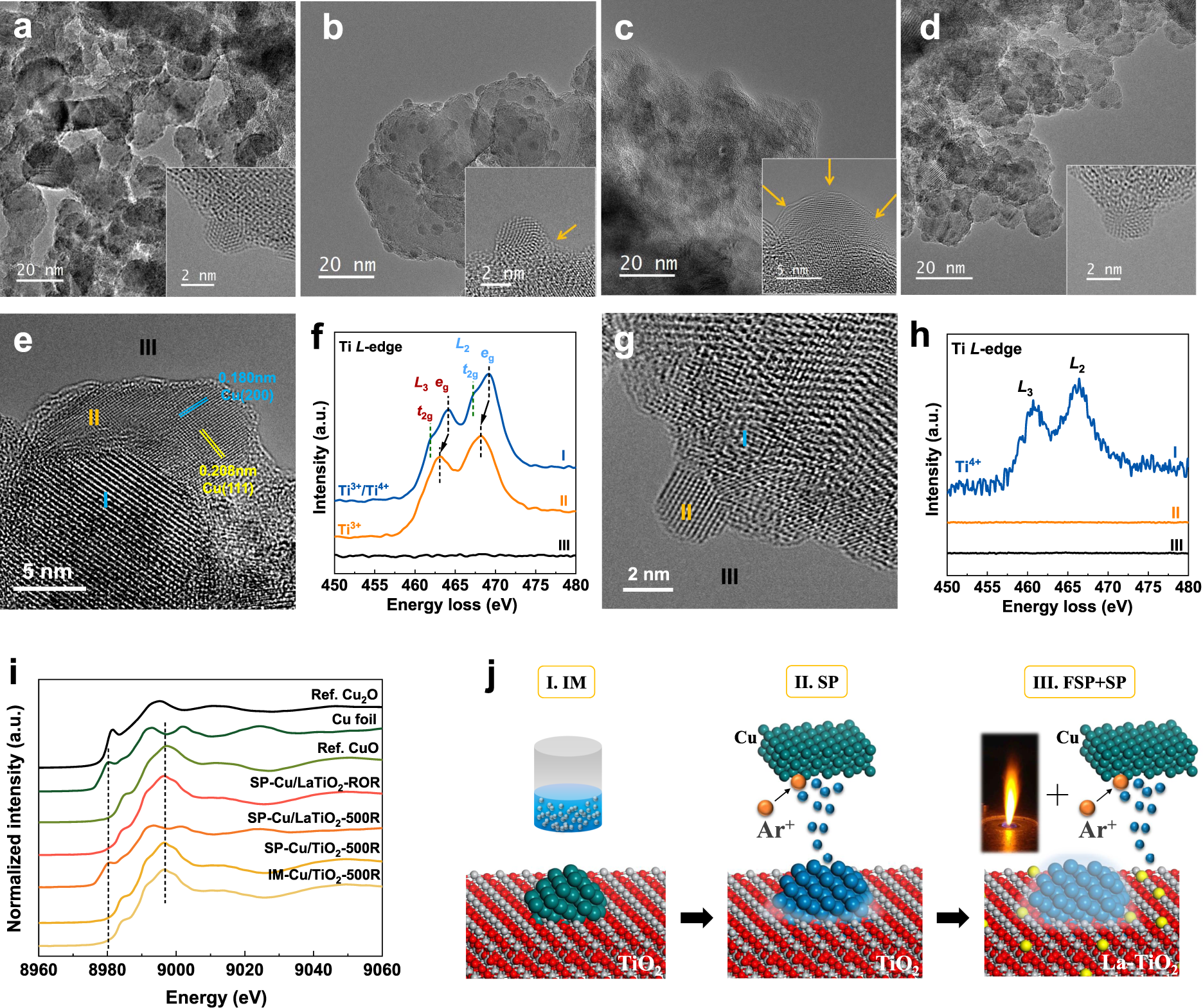



Ultra High Thermal Stability Of Sputtering Reconstructed Cu Based Catalysts Nature Communications
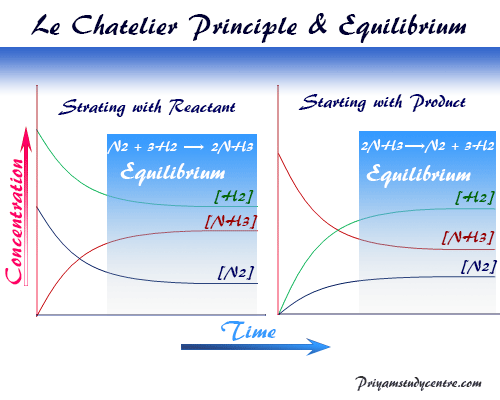
According to Le Chatelier's principle, an increase in temperature shifts the equilibrium to a backward reaction and decreases the equilibrium concentration of ammonia in ammoniaReaction rates generally increase with an increase in temperature Consequently, equilibrium is established sooner Also, the value of the equilibrium constant Kc varies with temperature TheLe Chatelier's principle predicts that the equilibrium will move to the right with an increase in temperature as the forward reaction is endothermic The brown colour intensifies The




Le Chȃtelier S Principle Changing Temperature Equilibrium Ap Chemistry Khan Academy Youtube




On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times The Journal Of Chemical Physics Vol 131 No 21
Le Chatelier's principle addresses how an equilibrium shifts when the conditions of an equilibrium are changed The direction of shift can be predicted for changes in concentrations, Le Chatelier's Principle on Change of Temperature log(K 1 /K 2) = ΔHº/R (1/T 2 1/T 1) Example N 2 (g) 3H 2 (g) 2NH 3 (g) ΔH = 92kJ Case1 Exothermic Reaction In the With this assumption, we can let Δ G = − R T ⋅ ln K eq = − 351 k J / m o l We know Δ G, R = 1 × 10 − 3 and let's say the data is given for room temperature, so T = 298 K Solving
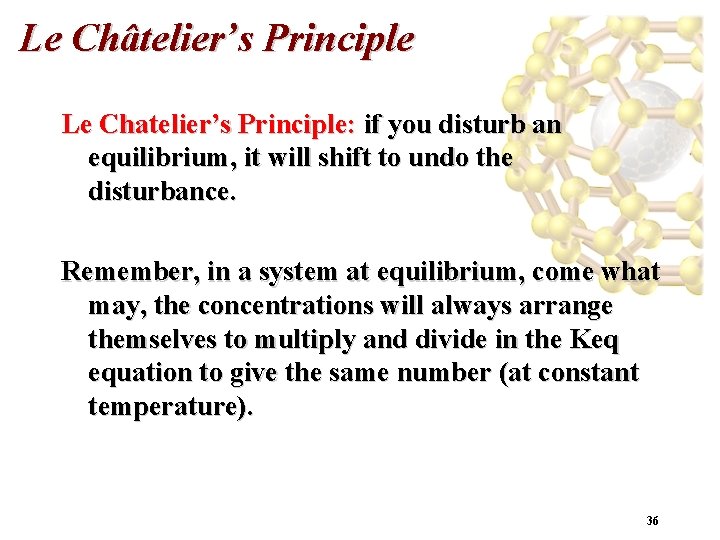



Chemical Equilibrium Save Paper And Ink When You




Le Chatelier S Principle Ppt Download
Find part 1, the otheApplying Le Chatelier's Principle to Saturated Solutions Solubility tables like the one above, tell us the maximum mass of solute that will dissolve in a given mass of solvent under certain Le Chatelier and Karl Ferdinand Braun independently proposed the principle, which is also known as Chatelier's principle or the equilibrium law The law may be stated When a
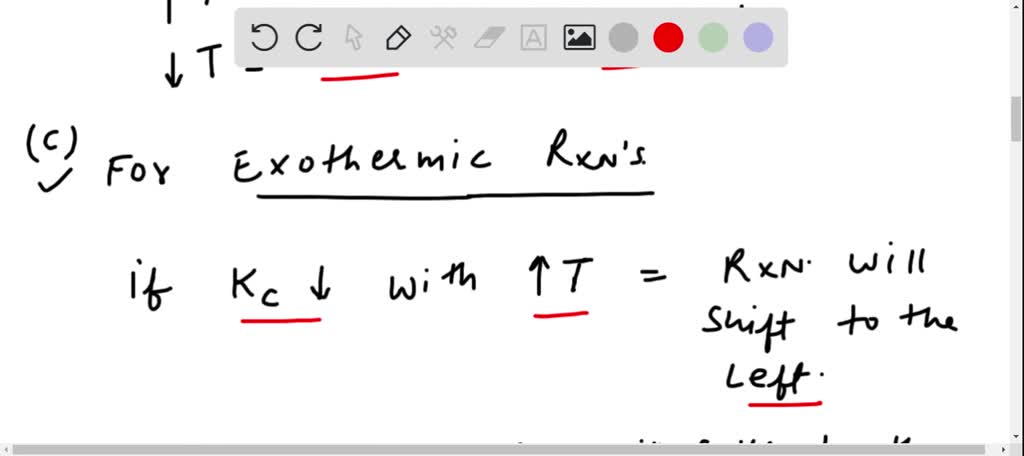



Solved For Each Of The Questions Four Choices Have Been Provided Select The Correct Alternative According To Le Chatelier S Principle A An Increase In Pressure Always Causes A Change In Position Of Equilibrium




Le Chatelier S Principle Youtube
According to Le Chatelier's principle, the influence of temperature on chemical equilibrium is defined by the sign of H in the reaction The heat of reaction is what determines the influence ofFor purposes of applying Le Châtelier's principle, heat, q, may be viewed as a product Raising the temperature of the system is akin to increasing the amount of a product, and so the equilibrium
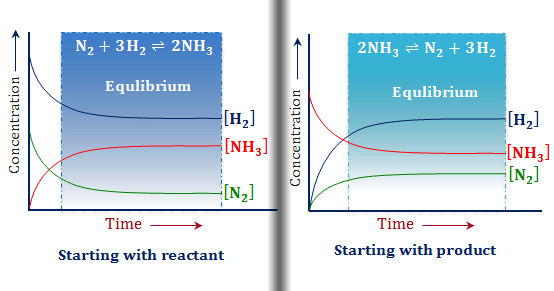



Le Chatelier Principle Equilibrium Reactions By Chemistry Topics Medium




Colloidal Ald Grown Core Shell Cdse Cds Nanoplatelets As Seen By Dnp Enhanced Pass Pieta Nmr Spectroscopy Nano Letters




Le Chatelier S Principle Ppt Download




Le Chatelier S Principle Equation Temperature Pressure Examples Video Lesson Transcript Study Com




Yolk Shell Nanocapsule Catalysts As Nanoreactors With Various Shell Structures And Their Diffusion Effect On The Co2 Reforming Of Methane Acs Applied Materials Interfaces



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts




Inhibition Of Temperature Runaway Phenomenon In The Sabatier Process Using Bed Dilution Structure Lbm Dem Simulation Lin 21 Aiche Journal Wiley Online Library




Chemical Equilibrium Analysis Of Hydrogen Production From Shale Gas Using Sorption Enhanced Chemical Looping Steam Reforming Sciencedirect



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts
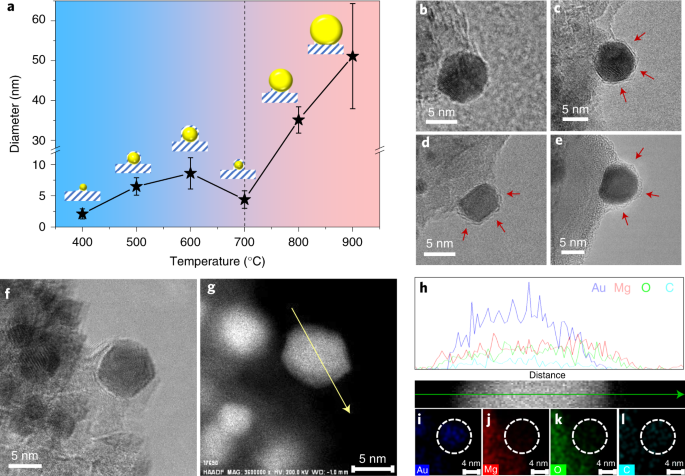



Strong Metal Support Interactions On Gold Nanoparticle Catalysts Achieved Through Le Chatelier S Principle Nature Catalysis
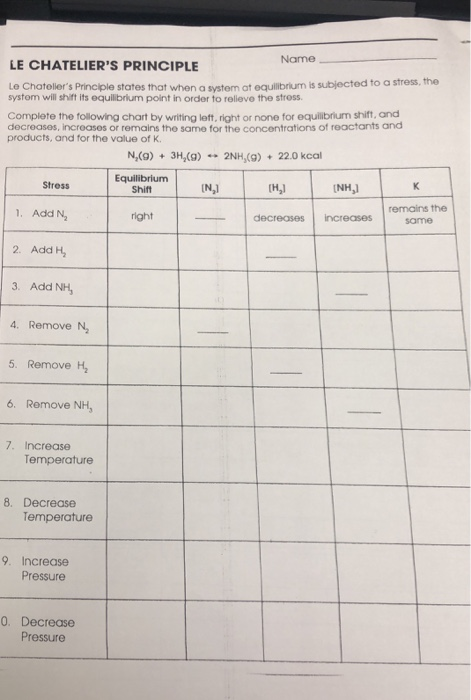



Solved Name Le Chatelier S Principle Le Chotolier S Chegg Com



Le Chatelier S Principle Temperature Youtube




7 1 Le Chatelier S Principle Changes In Temperature Sl Youtube




Hsc Chemistry Le Chatelier Principle And Equilibrium Guide



Le Chatelier S Principle Ppt Download




Le Chatelier S Principle Definition Examples Rules Applications




A Demonstration Of Le Chatelier S Principle On The Nanoscale Acs Central Science
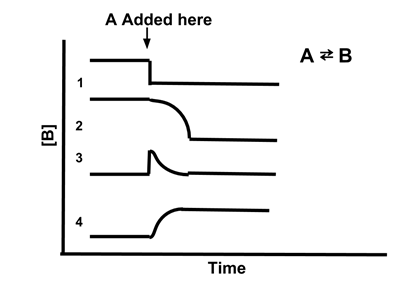



Le Chatelier S Principle




Le Chatelier S Principle Ppt Download



Le Chatelier Principle Equilibrium Reactions By Chemistry Topics Medium




Inert Effect On Chemical Equilibrium Youtube
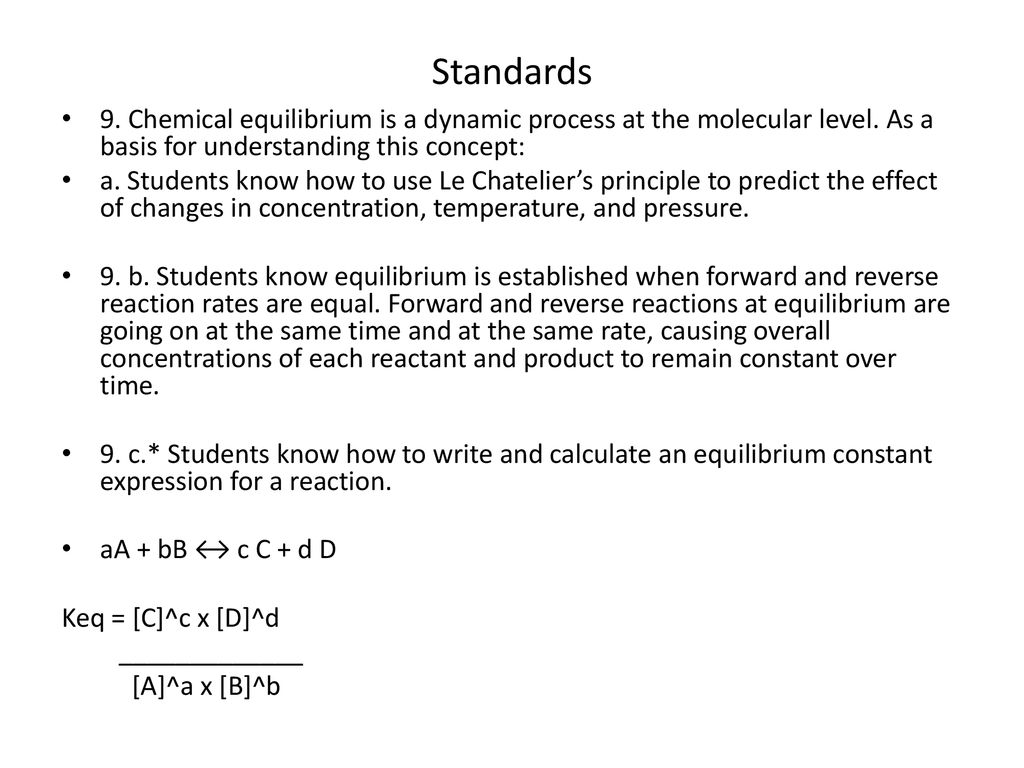



Le Chatelier S Principle Ppt Download




7 1 Le Chatelier S Principle Changes In Temperature Sl Youtube




Pdf On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times




Le Chatelier S Principle Analytical Chemistry Video Clutch Prep




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times The Journal Of Chemical Physics Vol 131 No 21
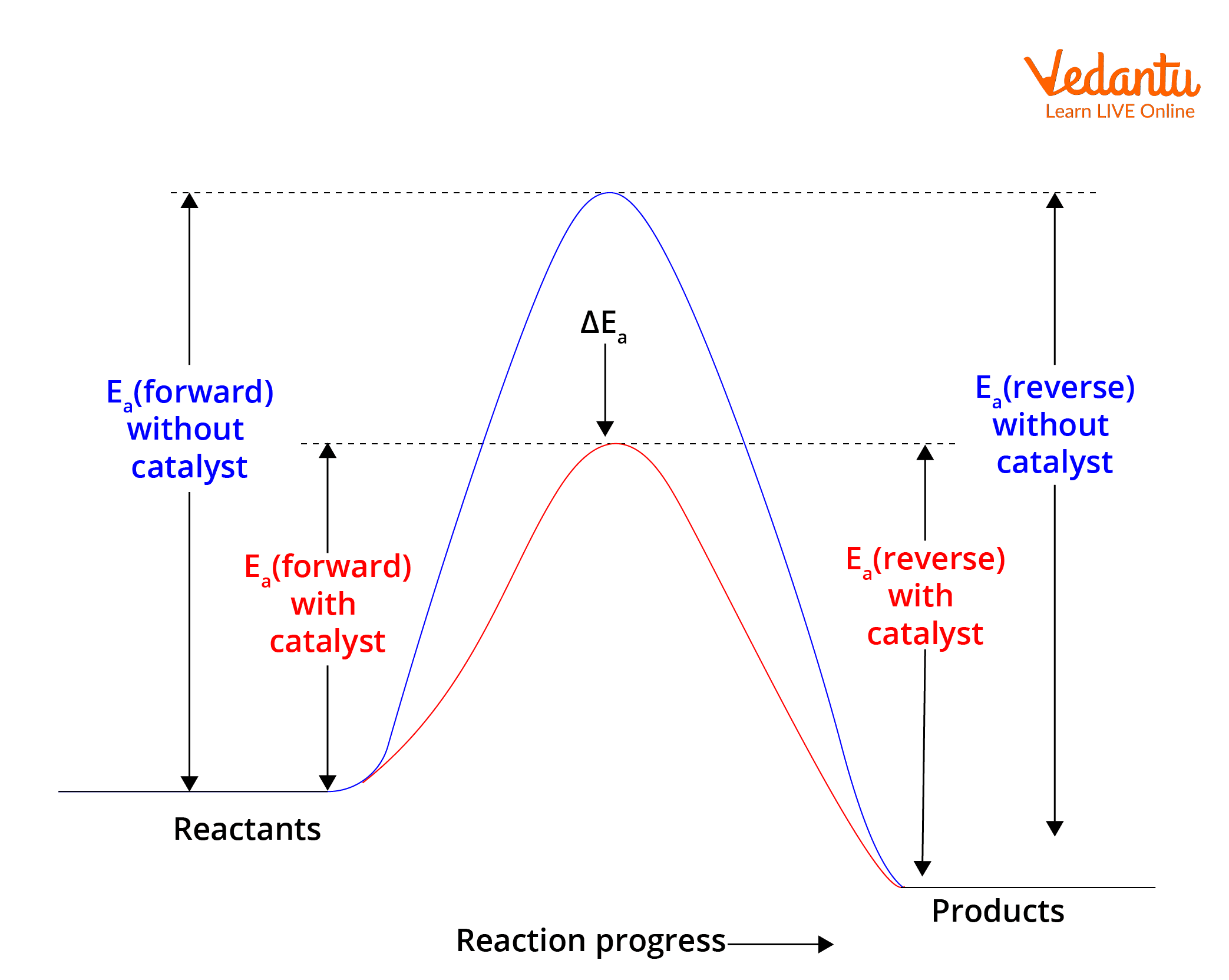



Le Chatelier S Principle Laws Principle Example And Faqs For Jee
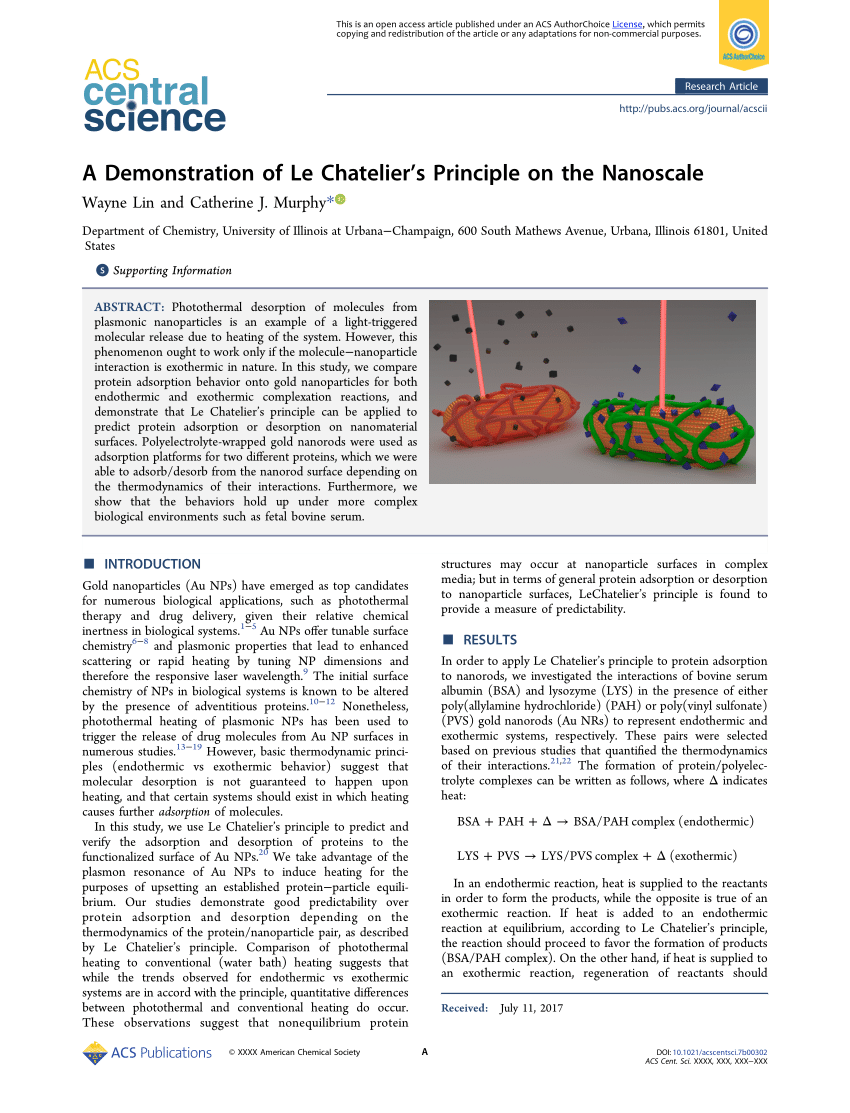



Pdf A Demonstration Of Le Chatelier S Principle On The Nanoscale




Le Chatelier S Principle Definition Examples Rules Applications




Solution Le Chatelier S Principle Studypool



Strong Metal Support Interactions On Gold Nanoparticle Catalysts Achieved Through Le Chatelier S Principle Nature Catalysis




Physical Chemistry How To Explain Disagreement Between Le Chatelier S Principle And The Simplified Gibbs Free Energy Equation Chemistry Stack Exchange



Le Chatelier S Principle And Temperature Changes Pt 10 Youtube



Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download



Lechatelier Principle



2
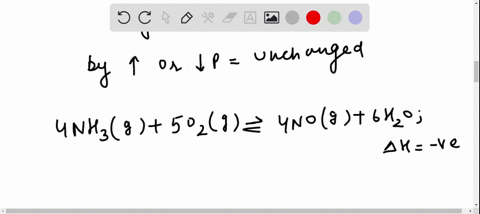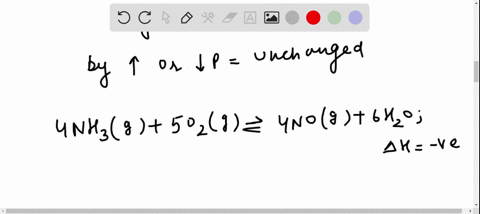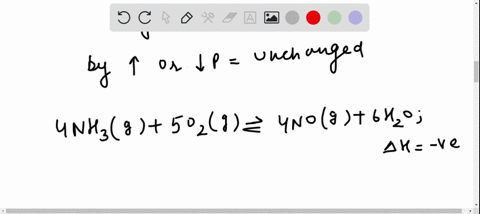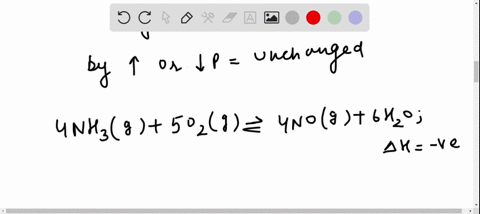



Solved For Each Of The Questions Four Choices Have Been Provided Select The Correct Alternative According To Le Chatelier S Principle A An Increase In Pressure Always Causes A Change In Position Of Equilibrium




Le Chatelier S Principle Worked Example Video Khan Academy




Acids And Bases Today S Topic Le Chatelier S Principle Law Of Mass Action Equilibrium And Dissociation Constants Ppt Download
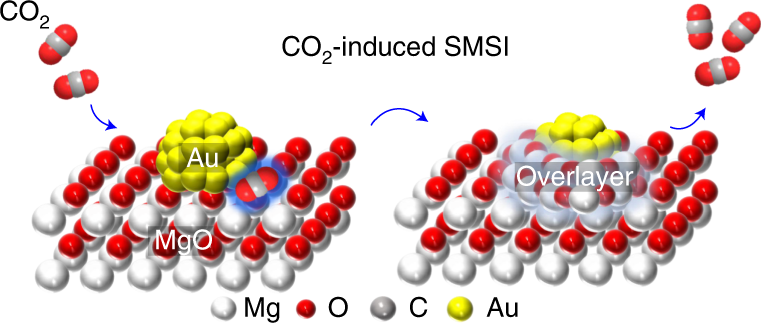



Strong Metal Support Interactions On Gold Nanoparticle Catalysts Achieved Through Le Chatelier S Principle Nature Catalysis




Le Chatelier S Principle




On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times The Journal Of Chemical Physics Vol 131 No 21



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts




Physical Chemistry How To Explain Disagreement Between Le Chatelier S Principle And The Simplified Gibbs Free Energy Equation Chemistry Stack Exchange




Le Chatelier S Principle Ppt Download




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




Chemistry Not Mystery Le Chatelier S Principle Temperature Change



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts




Use Le Chatelier S Principle To Predict Whether Each The Following Changes Causes The System To Shift In The Direction Of Products Or Reactants Homework Study Com




Super Dry Reforming Of Methane Intensifies Co2 Utilization Via Le Chatelier S Principle Science



Solved For Each Of The Questions Four Choices Have Been Provided Select The Correct Alternative According To Le Chatelier S Principle A An Increase In Pressure Always Causes A Change In Position Of Equilibrium




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




Understanding Le Chatelier S Principle Youtube




Direct Conversion Of Syngas Produced From Steam Reforming Of Toluene Into Methane Over A Ni Biochar Catalyst Acs Sustainable Chemistry Engineering
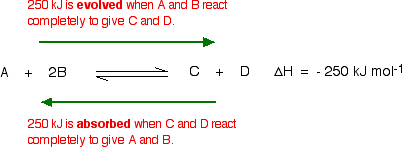



Le Chatelier S Principle




Reversible Reactions Equilibrium And Le Chatelier S Principle Compound Interest
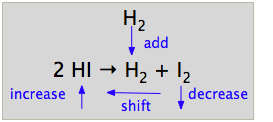



11 2 Le Chatelier S Principle Chemistry Libretexts



Solved Question 4 Of 7 Set An To 0 And Ah To Exo In The Le Chegg Com




Tips For Le Chateliers Principle Concept Chemistry Video By Brightstorm




Le Chatelier S Principle Ck 12 Foundation



Le Chatelier S Principle




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




Science Kit Equilibrium Le Chatelier S Principle For Teaching How Stress Effects Chemical Systems Hands On Lab Activity Materials For 15 Setups Innovating Science Walmart Com



1




13 05 Le Chatelier S Principle Youtube



1




7 1 Le Chatelier S Principle Temperature Sl Youtube




On Violations Of Le Chatelier S Principle For A Temperature Change In Small Systems Observed For Short Times The Journal Of Chemical Physics Vol 131 No 21




Le Chatelier S Principle Temperature Change Youtube
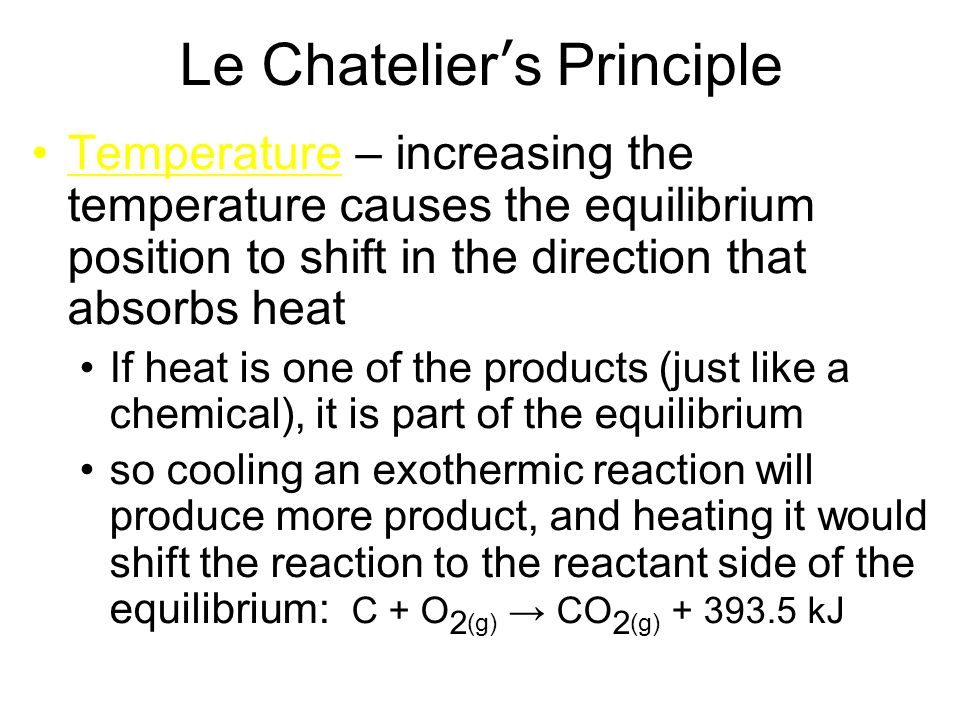



Notes Equilibrium Le Chatelier S Principle 18 1 18 2 Ppt Video Online Download




J3y7u4fjtfabvm



1



Chemistry Equilibrium Le Chateliers Principle Temperature And Catalysts




Lechatelier S Principle Equilibrium Lechatelier S Principle Co 2 Cao Caco 3 Chicken Breath Food Egg Shell I Wish I Had Sweat Glands As Temperature Ppt Download




Ocean Chemistry American Chemical Society
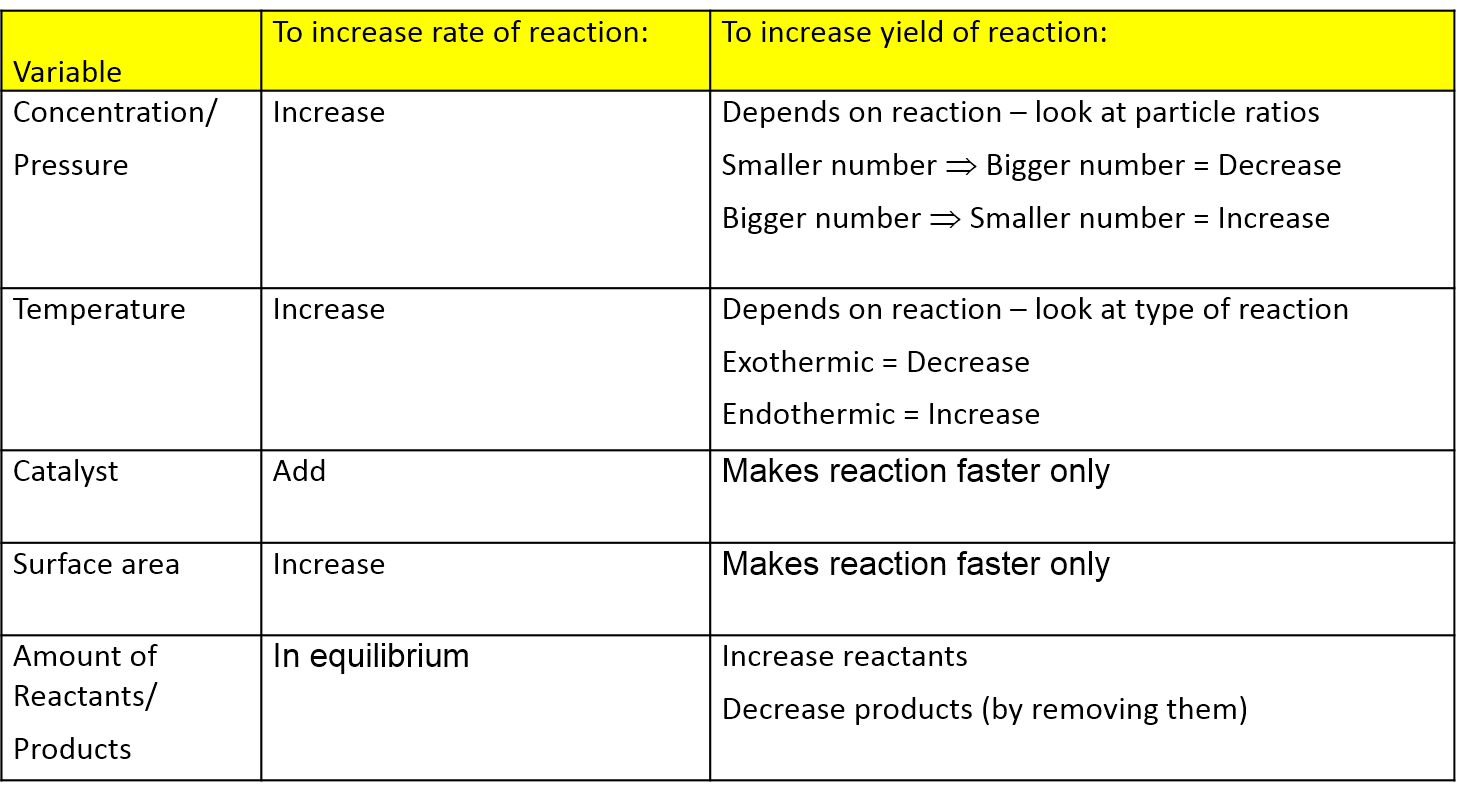



Le Chatelier S Principle Vce Chemistry




15 6 Altering Equilibrium Conditions Le Chatelier S Principle Chemistry Libretexts



Le Chatelier Principle Equilibrium Reactions By Chemistry Topics Medium




Le Chatelier S Principle Changing Temperature Chemistry Jove



0 件のコメント:
コメントを投稿